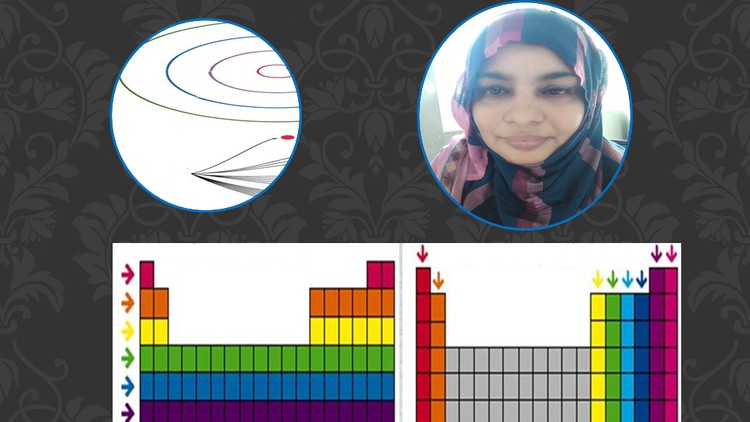
Electronic Configuration by Auf Bau Principle.
☑ Do you know structure of an atom?
☑ Do you the fundamentals particles of an atom?
☑ Where electrons, protons and neutrons reside in an atom?
☑ Can you locate the position of an electron in an atom?
☑ What are Shells and Sub-Shells?
☑ How electrons accommodate in shell? and sub-shell?
☑ Do you know maximum capacity of shells and sub-shells?
☑ What is Auf-Bau principle?
☑ How many sub-shells are there?
☑ Are all the shells can accommodate equal number of electrons?
☑ Practice to write electronic configuration.
This course is design for chemistry students of intermediate level or A-level who need help to write electronic configuration and find out the valence shell electronic configuration of elements from the periodic table.
This course is consist of four module each module further sub divided into three steps i.e. basic information review and a short quiz and finally a detailed quiz to complete this course.
Module – 1
1-Atomic Structure.
1.1 Position of electrons protons and neutron in an atom.
1.2 Nuclear and extra nuclear part of an atom.
1.3 Shells and Sub-Shells.
1.4 Presence of sub-shells in a shell.
1.5 Maximum accommodation of electrons within a shell.
1.6 Types of sub-shells their names with capacity.
Module – 2
2- Shape of periodic table.
2.1- Periodic Table.
2.2 Groups and periods blocks in periodic table.
2.3 Blocks in periodic table and their position in periodic table.
Module – 3
3 – On construction principle.
3.1 How electrons are distributed in its atomic orbitals.(s,p,d & f)
3.2 What are energy levels?
3.3 Write electronic configuration of different elements.
Module -4
4- Importance of electronic configuration
4.1 Valance shell electronic configuration.
4.2 Identify atomic properties.(Groups, periods, block and chemical properties etc.
Module -5
5- How we can write Electronic Configuration (PRACTICE…)
5.1 Electronic configuration of s-block elements.
5.2 Electronic configuration of p-block elements.
5.3 Electronic configuration of d-block elements.
5.4 Electronic configuration of f-block elements.
English
Language
Module -1
Atomic Structure.(Atoms are the basic units of matter)
Module – 2
2- Shape of periodic Table
Module – 3
Auf Bau Principle.
Module – 4
Importance of electronic configuration.
Module – 5
How we can write Electronic Configuration (PRACTICE…)